Early Detection of Ovarian Cancer Is Crucial
Early Detection of Ovarian Cancer
Ovarian cancer is a devastating disease and is often diagnosed too late. It is the deadliest gynecological cancer and the
~5th leading cause of cancer-related deaths in women1
Early detection may make all the difference
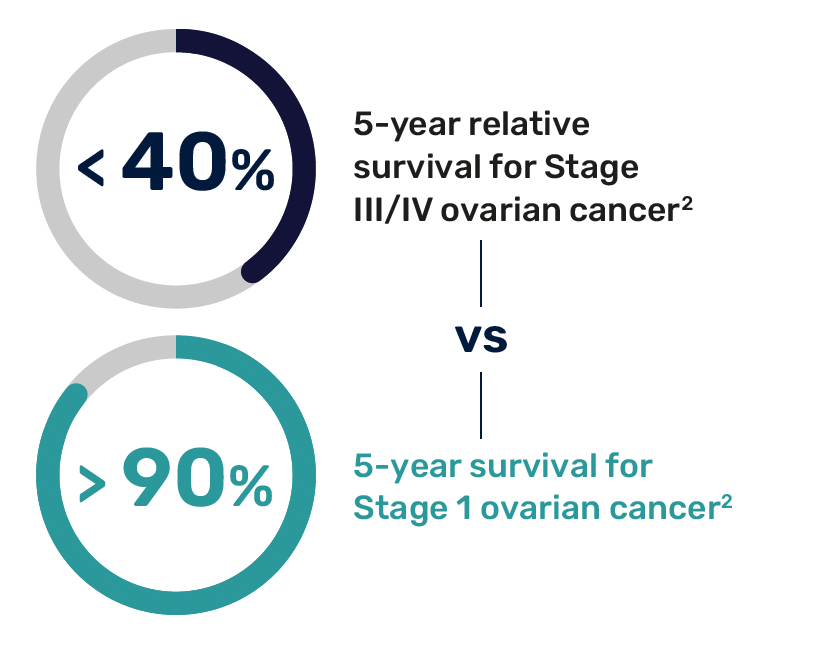
Significant Need for High-Risk Patients
Women at high risk for ovarian cancer include those with inherited genetic mutations, such as BRCA1 and BRCA2, Lynch syndrome, or a strong family history of ovarian, breast, uterine, or colorectal cancer, as well as other significant risk factors.3

Limited Effectiveness of Current Early Detection Methods
A major challenge in early ovarian cancer detection is the limited effectiveness of current screening methods, such as the CA-125 blood test and transvaginal ultrasound. These methods often fail to detect cancer early and produce false positives, leading to unnecessary surgeries and increased anxiety.4,5
Current professional guidelines recognize the limitations of existing ovarian cancer early detection methods, underscoring the need for more effective and reliable tests, particularly for high-risk women.6
Routine ovarian cancer screening is not recommended for average-risk women;4 however, for those at high risk, specific assessments are advised:
• Women with a personal or family history of breast, ovarian, or related cancers, particularly those with BRCA1/2 mutations, should be assessed for hereditary cancer risk.6
• Both NCCN and ACOG state that in cases where certain women are deemed high-risk, periodic testing for ovarian cancer (transvaginal ultrasound, CA-125) can be considered as part of a broader risk-reduction strategy.6,7
A breakthrough innovation for the early detection of ovarian cancer
The Avantect Ovarian Cancer Test is a novel, cell-free DNA-based blood test to aid in earlier diagnosis in women at high risk for developing ovarian cancer, offering a greater chance for improved survival.

Important information
The Avantect Ovarian Cancer Test is an early detection test. The test does not establish a diagnosis of ovarian cancer, and results should be considered in the context of other clinical criteria. A definitive diagnosis of cancer is rendered by clinical providers through a combined use of diagnostic testing, imaging, biopsy, and pathological findings. Not all ovarian cancers will be detected. Some patients with ovarian cancer may have a “Signal not detected” result. Some patients without ovarian cancer may have a “Signal detected” result. False-negative and false-positive results are possible. A “Signal not detected” result does not guarantee that no ovarian cancer is present. In some cases, no result is obtained. While this is very uncommon, it may be caused by shipping delays or when there is not enough cell-free DNA for the test in the patient’s blood. If this happens, additional blood samples may be required to produce a patient result.
The test was developed in the ClearNote Health CLIA-certified (CLIA# 05D2249973) and CAP-accredited (CAP# 9219174) laboratory and has not been cleared or approved by the US Food and Drug Administration (FDA).
References and notes
- Cronin et al. “Annual report to the nation on the status of cancer, part 1: National cancer statistics” Cancer. 2022;128:4251–4284
- Cancer Stat Facts: Ovarian Cancer. (Accessed 1/25/2024, at https://seer.cancer.gov/statfacts/html/ovary.html.)
- CDC Ovarian Cancer Risk Factors. https://www.cdc.gov/ovarian-cancer/risk-factors/index.html
- Screening for Ovarian Cancer-US Preventive Services Task Force Recommendation Statement. (Accessed 4/23/2024, at https://jamanetwork.com/journals/jama/fullarticle/2672638.)
- Dullens B, de Putter R, Lambertini M, et al. Cancer Surveillance in Healthy Carriers of Germline Pathogenic Variants in BRCA1/2: A Review of Secondary Prevention Guidelines. J Oncol 2020;2020:9873954.
- ACOG Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol. 2017 Sep;130(3):e110-e126.
- Daly et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Jan 6;19(1):77-102.